By Florence Sessoms
Urea, coated urea, ammonium sulfate, and ammonium nitrate are the most common source of nitrogen (N) in fertilizers applied in lawns; this indicates that ammonium (NH4+) is the major source of inorganic nitrogen artificially applied in these systems. Ammonium can be taken up by plants and also used by different types of nitrifiers resulting in its conversion into nitrate (NO3-). See previous blog about using turfgrass root exudates to decrease nitrogen losses. Nitrate, a very mobile anion, is an additional source of inorganic N for plants or can be lost by leaching or through denitrification. Biological nitrification inhibition (BNI) is the ability of plant roots to produce and exude compounds into the soil that block the nitrification. This natural process reduces nitrogen losses, and has a similar nitrogen-saving effect as several chemical additives that have been commercially available for many years, including dicyandiamide (DCD), 3,4-dimethylpyrazole phosphate (DMPP) and nitrapyrin.
Turf-type perennial ryegrass has been used for decades in various turfgrass systems. This species can be found in residential lawns, athletic fields, golf courses, and parks. Perennial ryegrass qualities include fast germination (less than 10 days), wear tolerance and smooth dark-green leaves making it an ideal recreational turf. However, modern cultivars require high fertilization inputs (2-5lb of N/1000 sq feet per year) to maintain high turfgrass quality. In fact, lower fertility regimes will result in increased leaf-blade shredding (1), higher stemminess (2) and the appearance of disease such as red thread (3), dollar spot (4) or rust (5).
So far, biological nitrification inhibition capacity has not been evaluated in perennial ryegrass but could be an exciting trait for the development of new cultivars with lower nitrogen requirement. I am part of a team with Dr. Bryan Emmet from USDA-ARS, Dr. Rod Venterea (UMN Department of Soil, Water and Climate) in which we are evaluating the BNI capacity of perennial ryegrass accessions.
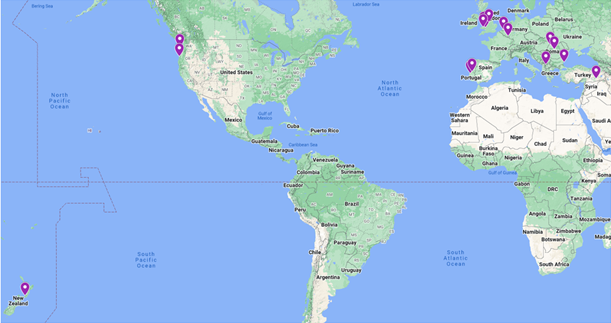
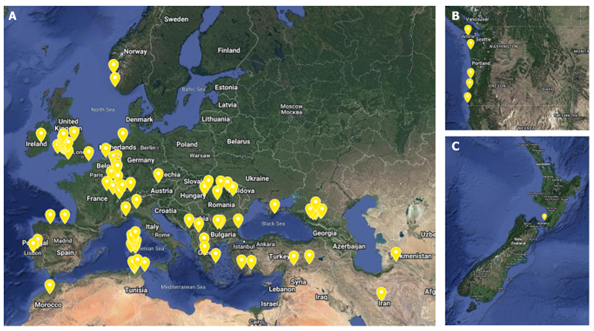
A total of 92 perennial ryegrass wild accessions were ordered from the U.S. National Plant Germplasm System; these accessions were chosen by location to capture wide geographic, environmental, and genetic diversity. The location of all accessions tested can be seen in Figure 1.
For each accession, several seeds were sown in small trays containing a soilless germination mix and moved into a mist-house for germination (Figure 2A). Several accessions had no germination resulting in only 82 accessions being successfully tested. Young seedlings (Figure 2B) were cultivated for 5 weeks, clipped and watered as needed. Plants were fertilized with a modified Hoagland solution (6) containing 1 mM of ammonium as the only source of added N. This low concentration of ammonium has been shown to stimulate plants into producing and secreting inhibitors of nitrification, as the plant root system and the bacterial nitrifier population will compete for the low amount of nitrogen present in the soil. At the end of the experiment, the rhizosphere soil was harvested (Figure 2C) and used to determine potential nitrification inhibition.
Shaken soil slurry experiments are generally used to determine the potential nitrification rate (PNR) of soils (7). This methodology and its result are associated with the size of the nitrifier population. We hypothesized that in our experimental set-up, potential BNI exudates from perennial ryegrass root systems would be secreted and would accumulate in the rhizosphere soil, inhibiting the nitrifier population’s growth, leading to reduced nitrification rate. This methodology was successfully used by O’Sullivan et al in 2017 (8). For each plant, several grams of rhizosphere soil was transferred in a Mason jar (Figure 3A), and a buffer solution containing ammonium (new source of N for the nitrifiers) was added (Figure 3B) as described in (7). Several milliliters of the soil-slurry solution were pipetted at different time points to evaluate the NO3 accumulation (Figure 3C-D). These experiments contained several types of controls: soil only (unaltered PNR or 100% PNR), soybean (negative control- unknown BNI, PNR should be similar to soil only), sorghum (positive control- known BNI capacity, reduced PNR), and DCD (soil only+DCD, chemically reduced PNR).
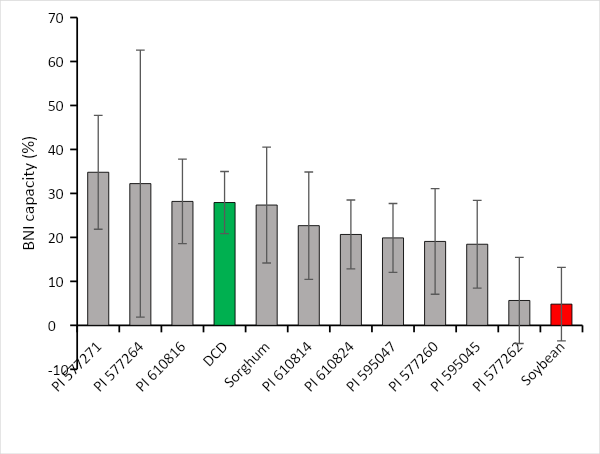
For each run, the potential BNI capacity (BNI) was calculated by comparing the soil only PNR (100% PNR and no BNI capacity) to the accession’s PNR. Most accessions possessed a higher BNI capacity than soybean (Figure 4). 16 accessions displayed a higher potential BNI capacity from the DCD and sorghum (Figure 4). Interestingly, these accessions originated from various locations such as the UK, US, Belgium, New Zealand, Spain, Portugal, Turkey (Figure 5), suggesting that high potential BNI capacity is not associated with geographical location and climate types.
Finally, based on our preliminary data, potential BNI capacity can greatly vary among accessions sampled in a similar location and within the genotypes of each accession. For example, in our experimental set-up, 10 perennial ryegrass accessions from Wales (UK) were evaluated for their BNI capacity (Figure 6). The results showed that in addition to the large variation in potential BNI capacity observed within an accession, accessions originating from a similar location, here Wales, could present very different potential BNI capacities.
What is next?
First of all, a few more runs will be performed to validate the potential BNI capacity of the accessions. More importantly, additional bioassay experiments need to be done to confirm that the lower PNR and high BNI originate from nitrifier’s inhibition.
Overall, these preliminary results are extremely exciting and reveal that several wild accessions of perennial ryegrass display potentially high BNI capacity. This trait needs to be confirmed, and could be incorporated in further breeding programs and cultivar development.
In conclusion
- This is the first evidence of potential BNI capacity in perennial ryegrass, a widely used recreational turfgrass.
- Accessions display a wide potential BNI capacity.
- Potential BNI capacity did not seem to be associated with specific geographical location.
References
(1) Gibeault, V. A., and Hanson, D. (1980). Perennial ryegrass mowing quality and appearance response to three nitrogen regimes. In J. B. Beard (Ed.), Proceedings of the 3rd International Turfgrass Research Conference (pp. 39–43). ASA, CSSA, SSSA.
(2) Braun, R. C., and Patton, A. J. (2022). Perennial ryegrass (Lolium perenne) culm and inflorescence density in lawns: Effects of nitrogen fertilization and scalping timing and height. Crop Science, 62:489–502. doi.org/10.1002/csc2.20665
(3) Qu,Y., Mohr, M. M., Bara, R. F., Smith, D. A., Szerszen, E., Bonos, S. A., and Meyer, W. A. (2015). Performance of perennial ryegrass cultivars and selections in New Jersey turf trials. In A. B. Gould (Ed.), 2015. Turfgrass (pp. 125–147). Atlantic City, NJ: Rutgers University.
(4) Dunn, J. H., Minner , D. D., and Bughrara, S.S. (1996). Clippings disposal and fertilization influence disease in perennial ryegrass. HortScience, 31:1180-1181.
(5) https://agsci.oregonstate.edu/beaverturf/perennial-ryegrass-lolium-perenne-l
(6) Hoagland, D. R, and Arnon, D. I.(1950). The water-culture method for growing plants without soil. Circ. 347. Univ. of Calif. Agric. Exp. Station, Berkley.
(7) Hart, S. C., Stark, J. M., Davidson, E. A, and Firestone, M. K. (1994). Methods of soil analysis: part 2—microbiological and biochemical properties. Soil Science Society of America, Madison, Wisconsin, USA.
(8) O'Sullivan, C. A., Whisson, K., Treble, K., Roper, M. M., Micin S.F., and Ward, P. R. (2017). Biological nitrification inhibition by weeds, wild radish, brome grass, wild oats and annual ryegrass decrease nitrification rates in their rhizospheres. Crop Pasture Sci., 68 (2017), pp. 798-804.


![Sessoms - Fig 4 Figure is a bar graph of average potential BNI capacity of 82 perennial ryegrass accessions and the different controls; DCD’s BNI capacity is colored in green, soybean’s BNI capacity is colored in red. Figure 4. Average potential BNI capacity of 82 perennial ryegrass accessions and the different controls; DCD’s BNI capacity is colored in green, soybean’s BNI capacity is colored in red. [Click and drag to move] ](/sites/turf.umn.edu/files/2022-09/Picture4.png)