By Florence Sessoms
Soil microbiomes are already known to be involved in nutrient cycling and allocation to plants. In my previous blogs, I described how the presence of mycorrhizal hyphal network in soil can provide certain nutrients (especially P and N) into plant root systems. Today, instead of focusing on fungi, I would like to discuss certain bacteria, their importance in the soil nitrogen cycle, and how plants can control them.
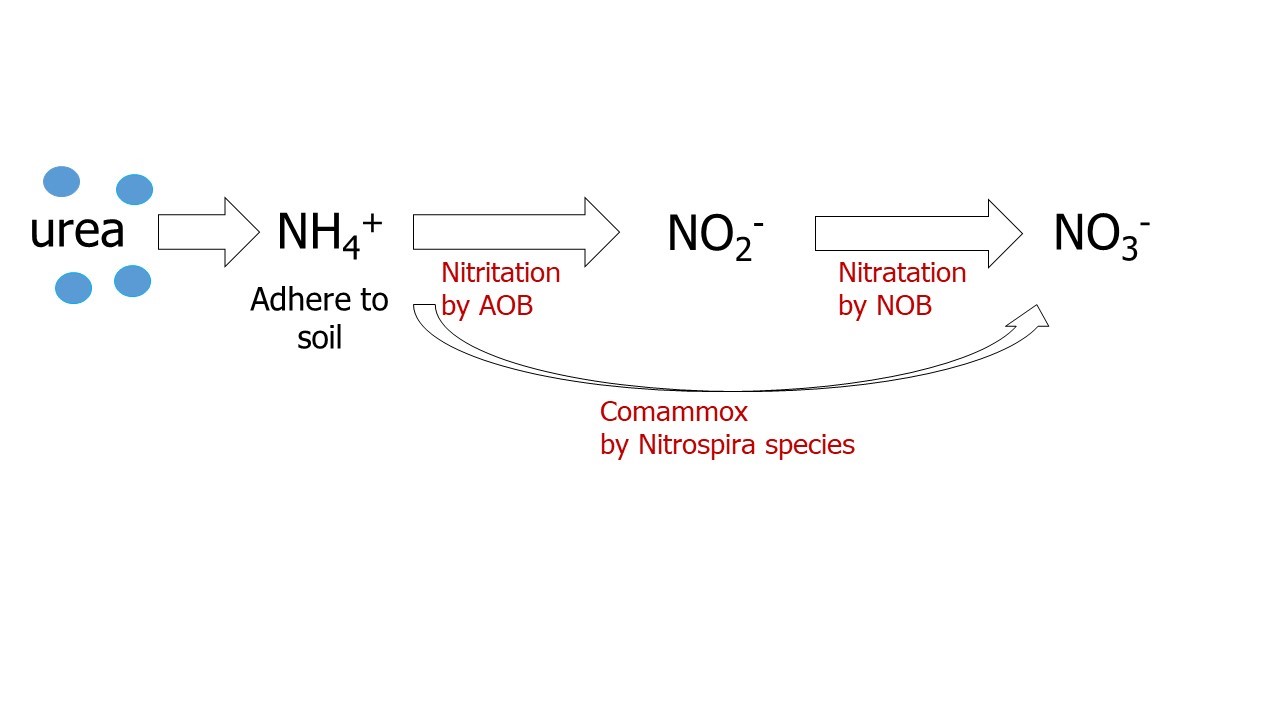
Soon after nitrogen fertilizer (urea or ammonium fertilizers) application, ammonium (NH4+) will undergo nitrification. This process of nitrification requires two steps (Figure 1), whereby ammonia (NH3) is oxidized into nitrate (NO3-) by different types of bacteria. The first process involves Nitrosomonas species, also called ammonia-oxidizing bacteria (AOB) that catalyze the transformation of NH3 into nitrite (NO2-); the second process involves Nitrobacter species, also called nitrite-oxidizing bacteria (NOB) that convert nitrite into nitrate. These two reactions demand different enzymes. Some microorganisms can directly oxidize ammonium into nitrate, a process call comammox (complete ammonia oxidation); this has been observed in Nitrospira, which possesses all the enzymes necessary for the two steps of nitrification.
Nitrate, because of its negative charge, is very mobile in soil and can be lost by leaching. Additionally, nitrate denitrification in wet anaerobic conditions (after heavy rainfall, for example) is known to produce nitrous oxide, a greenhouse gas 300 times more potent than CO2. These nitrogen losses form an important environmental problem especially for water bodies (lake, rivers, and estuaries) and the ozone layer.
Biological nitrification inhibition (BNI) is the ability of certain plant roots to produce and exude compounds into the soil which block the first step of nitrification. This natural process reduces nitrogen loss, and has a similar action of several chemical additives that have been commercially available for many years, including dicyandiamide (DCD), 3,4-dimethylpyrazole phosphate (DMPP) and nitrapyrin.
Biological nitrification inhibition was first found in hydroponic experiments in the tropical grass Bracharia humidicola (Subbarao et al., 2006a). However, additional experiments showed that secreted BNI compounds from B. humidicola could last up to 4 months in soils, indicating their stable nature when compared to the chemical nitrification inhibitors (Karwat et al., 2017). Pre-cropping B. humdicola with corn resulted in maize yield increases in the first year but not during the following years (Karwat et al., 2017). Recent publications on BNI have focused on wheat and rice, two species that were previously thought to lack BNI capacity (Subbarao et al., 2006b). Researchers screened 19 rice varieties and 96 wheat landraces grown under low ammonium conditions to favor the formation and secretion of BNI compounds (O’Sullivan et al., 2016; Sun et al., 2016); approximately 80% of the rice varieties and only 28% of the wheat landraces produced BNI compounds, but both of these studies showed a large genotypic variability in the secretion of BNI root exudates. Altogether, BNI may be an exciting new tool to increase nitrogen use efficiency and reduce N losses (Coskun et al., 2017) from lawns and a very attractive low-cost and localized alternative to the application of commercial inhibitors (O’Sullivan et al., 2016).
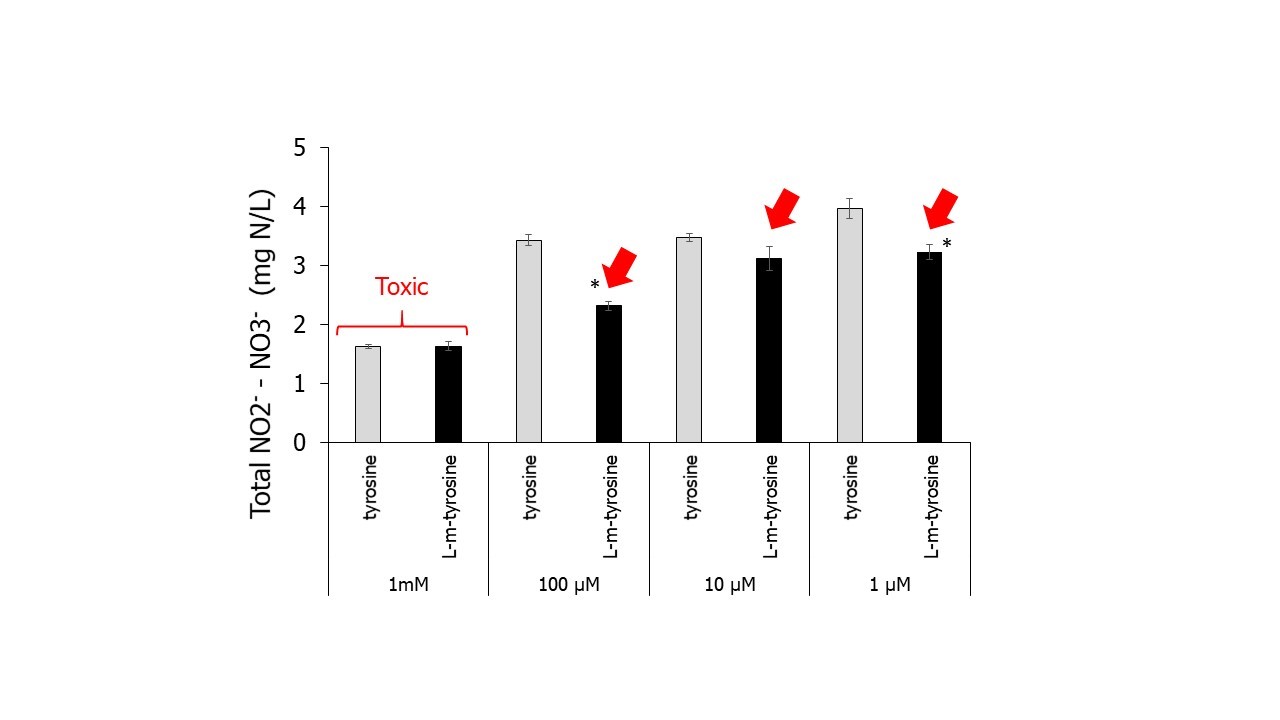
The fine fescues consist of 5 different species: hard fescue (Festuca brevipila), sheep fescue (Festuca ovina), Chewings fescue (Festuca rubra ssp. commutata), slender creeping red fescue (Festuca rubra ssp. littoralis), and strong creeping red fescue (Festuca rubra ssp. rubra) that are known to require lower levels of nitrogen inputs when used as lawn grasses. In addition to their reduced fertilization and irrigation needs, fine fescues have the ability of stabilizing soil nitrification rate (Banard et al., 2004) and one of the possible mechanisms being potential secretion of BNI compounds. Recent results from our lab found that L-m-tyrosine, a component of fine fescue root exudates that happens to also be associated with allelopathic effects on some weeds (Bertin et al., 2007), showed an ability to decrease total nitrate accumulation in a soil slurry experiment (Figure 2). This compound was already known to negatively affect different bacteria species (Aronson and Wermus, 1965).
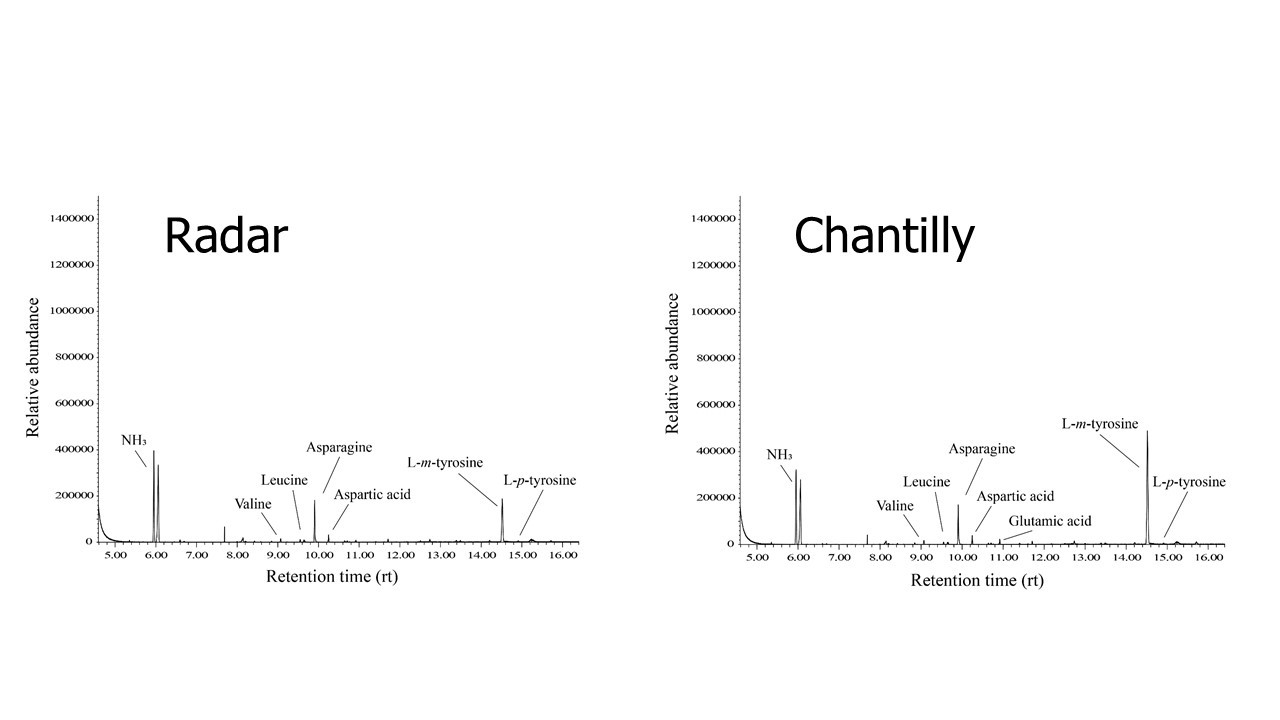
Further experiments with ‘Chantilly’ strong creeping red fescue and ‘Radar’ Chewings fescue led to the observation that L-m-tyrosine was present within their roots, and L-m-tyrosine was relatively the most abundant amino acid (Figure 3). However we have not demonstrated yet their ability to secrete L-m-tyrosine into the ground and further research.
In summary, recent research suggests that BNI may be an exciting new tool to increase nitrogen use efficiency and reduce N losses in turfgrass. Our long term objective, with this exciting and innovative research, is to enhance soil nitrogen conservation in urban lawns by focusing on reducing nitrogen input requirements through the use of fine fescue cultivars with BNI ability.
References
Aronson and Wermus (1965). Effects of m-Tyrosine on growth and sporulation of Bacillus species. Journal of Bacteriology, 90 (1): 38-46.
Barnard et al, (2004). Dynamics of nitrifying activities, denitrifying activities and nitrogen in grassland mesocosms as altered by elevated CO2. New Phytologist, 162: 365–376.
Bertin et al, (2007). Grass roots chemistry: meta-Tyrosine, an herbicidal nonprotein amino acid. Proceedings of the National Academy of Sciences, 104 (43): 16964-16969, DOI: 10.1073/pnas.0707198104.
Coskun et al, (2017). Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nature Plants, 3, DOI: 10.1038/nplants.2017.74.
Karwat et al, (2017). Residual effect of BNI by Brachiaria humidicola pasture on nitrogen recovery and grain yield of subsequent maize. Plant and Soil, 420: 389–406, DOI 10.1007/s11104-017-3381-z.
O’Sullivan et al, (2016). Identification of several wheat landraces with biological nitrification inhibition capacity. Plant and Soil, 404: 61–74, DOI 10.1007/s11104-016-2822-4.
Subbarao et al, (2006a). A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant and Soil, DOI 10.1007/s11104-006-9094-3.
Subbarao et al, (2006b) Biological nitrification inhibition (BNI)—is it a widespread phenomenon? Plant and Soil, DOI 10.1007/s11104-006-9159-3.
Sun et al, (2016). Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytologist, 212: 646–656.