By Jonathan Cors
Practically everyone knows about genetically modified plants and other genetically modified organisms (GMOs). I have found that it can be quite fun to talk with others, explaining the pros and cons of this technology. However, what I’ve found is that while everyone knows that genetically modified plants exist, few people know about how one is made. As a graduate student, I’ve spent a lot of time fine tuning and developing methods for genetically modifying turfgrasses. When I first started, I didn’t fully comprehend how someone makes a genetically modified plant, and very quickly learned that it was much more difficult than I had imagined.
There are several ways to create a GMO. Before we get into plants, it’s important to first mention how this process works in bacteria. Transforming the bacteria E. coli is a very simple process and can be done in a matter of minutes by utilizing well studied methods such as a heat shock or chemical transformation. I won’t go into detail about these methods, as there is plenty of other sources that would do a better job, but in short, these methods rely upon breaking small holes into the membrane of the bacteria, allowing DNA to enter and thus transforming it. When it comes to plants, this process can’t be used, and a few hurdles need to be overcome.
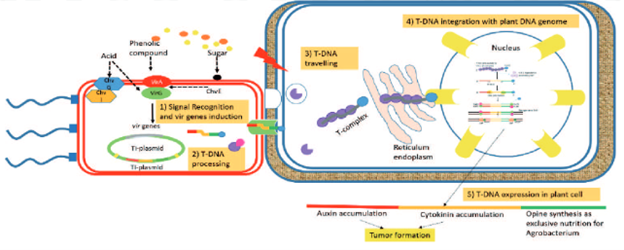
The first hurdle is that plant cells, unlike other eukaryotic or bacterial cells, have a cell wall covering the cells membrane. This cell wall is much stronger than the cell membrane and prohibits the creation of small holes that would allow for the DNA to enter. To get around this, plant molecular biologists can use a variety of methods, but one of the main methods practiced today is utilizing agrobacterium to insert the DNA into a cell. Agrobacteria are bacteria that specializes at injecting DNA into plants. These bacteria can be found infecting several plant species, creating the large crown galls that you can commonly see on trees for example. Researchers figured out how to exploit this process to move DNA from one organism to another using agrobacterium as the intermediary. Since this discovery, the approach has been improved and is now a plant molecular biologists’ primary tool for plant genetic transformation. By using agrobacterium, we can do the simple transformation methods just like with E. coli and insert a Ti-plasmid (DNA transfection vector) of our creation. The agrobacterium can then infect a plant cell, where a part of that Ti-plasmid, the transfer-DNA or T-DNA, will be inserted into the plant cells nucleus, effectively transforming it with our desired edit (Figure 1).
The second hurdle is the type of cell to transform. A plant is a large multicellular organism with thousands upon thousands of cells. As such, it’s important that we target the type of cells that would give us our desired outcome. If we were to target the leaf blade of turfgrass with agrobacterium, some number of cells in that leaf blade would be successfully transformed. However, this would cause only a mosaic of somatic cell transformations to occur, meaning that the transformation wouldn’t be passed onto the next generation of plants. Instead, as plant breeders we prefer to target embryonic cells, or cells that have their DNA pass onto the next generation, unlike other type of cells that don’t. The best choice of embryonic cells are usually meristem cells, or the cells that can differentiate into any cell type of the plant; these are similar to stem cells in humans. Targeting these cells will allow for the continued growth of the transformed cells as well as a high likelihood that some fraction of the offspring will also contain the genetic edit.
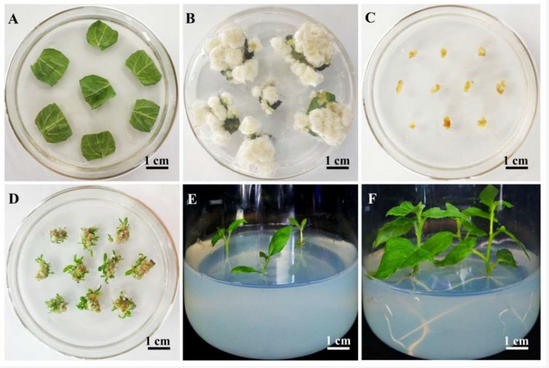
The third hurdle is getting access to a large number of these meristem cells. Under normal growing conditions, there are very few numbers of them. To solve this hurdle, callus culture is used. Callus culture is the process of plating plant tissue on media that contains certain growth hormones, usually a mixture of auxins and cytokines, to promote the cancerous growth of meristem cells. This results in small bundles of the cells that can then be dipped into an agrobacterium solution to transform them. After the transformation, you can than change the concentration of growth hormones to allow the meristem cells to differentiate back into a plant, effectively making a plant that is fully transformed and will pass on the edit to the next generation (Figure 2).
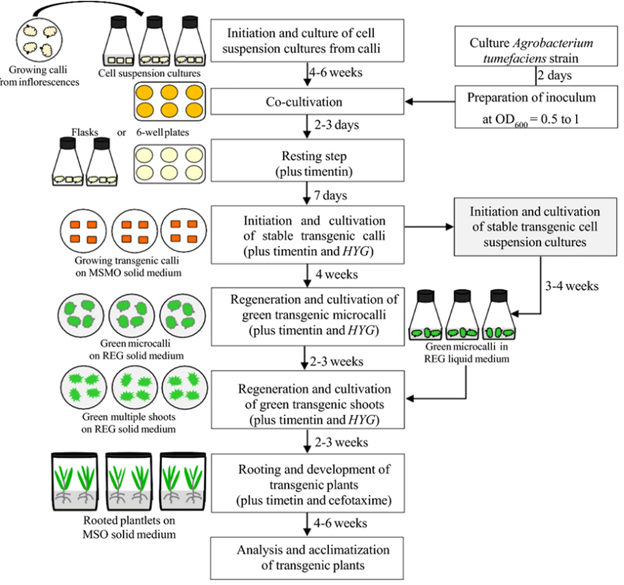
Figure 3 is an outline of all the steps involved in doing agrobacterium callus culture transformations, including how long each step takes. Callus culture and agrobacterium transformations are both lengthy processes. There are also several instances in each of these steps that something can go wrong, causing the loss of weeks or months of work. For instance, while generating callus it’s possible to get the plates or cell suspensions contaminated, which results in death of all the calli. After the transformation with agrobacterium, it’s possible for the agrobacterium to grow out of control, killing the callus. It’s possible for the transformation to not work, killing the callus on selection. The list goes on and on. However, if you work through all these issues, you can get successfully growing callus and recovered plants. That recovered plant can then be planted in soil, where it will grow and spread that genetic mutation on to the next generation. At that final growth stage, it can be characterized to see if the edits were correct and have the desired effect. If not, the plant can be thrown out and the whole process starts over once again.
While this process is certainly rather difficult, it’s currently one of the most common ways plant transformations are done. Many researchers are attempting to find ways to speed up this process, myself included. For instance, for a small number of species of plants, you can skip the callus induction and do a process called floral dip, where instead you simply infect seeds with the agrobacterium causing some number of them to be successfully genetically modified. Either way, once you have the genetic modification, you can then use normal breeding methods to start producing more seed for further testing. Once the testing is done, and assuming all regulations are being followed, it can be ready for release into the market.
References
Pratiwi, R. A. , & Surya, M. I. (2020). Agrobacterium-Mediated Transformation. In (Ed.), Genetic Transformation in Crops. IntechOpen. https://doi.org/10.5772/intechopen.91132
Wen, S.-S., Ge, X.-L., Wang, R., Yang, H.-F., Bai, Y.-E., Guo, Y.-H., Zhang, J., Lu, M.-Z., Zhao, S.-T., & Wang, L.-Q. (2022). An Efficient Agrobacterium-Mediated Transformation Method for Hybrid Poplar 84K (Populus alba × P. glandulosa) Using Calli as Explants. International Journal of Molecular Sciences, 23(4), 2216. https://doi.org/10.3390/ijms23042216
Ondzighi-Assoume, C. A., Willis, J. D., Ouma, W. K., Allen, S. M., King, Z., Parrott, W. A., Liu, W., Burris, J. N., Lenaghan, S. C., & Stewart, C. N. (2019). Embryogenic cell suspensions for high-capacity genetic transformation and regeneration of switchgrass (Panicum virgatum L.). Biotechnology for Biofuels, 12(1), 290. https://doi.org/10.1186/s13068-019-1632-3