By Jonathan Cors
A while back, I wrote a blog post on how to genetically modify a turfgrass plant where I went in depth on a process called Agrobacterium callus culture transformation. Briefly, it’s the process of promoting the growth of plant cells that can become any part of the plant. These cells are then transformed by utilizing Agrobacterium. Within the Agrobacterium will be a gene of interest that we have transformed into the it, which the bacterium then takes and puts it into the plant cells. It’s a very long and difficult process, taking about 9 months to a year in total, and has several points where it can go wrong. Despite all that, it is one of the best ways to create a genetically modified turfgrass plant currently. However, I’m always on the look out to see if there are ways to improve the protocol, or if new protocols have come out that are better and should be switched to.
Recently, researchers at Corteva put out a paper in Nature titled “Leaf transformation for efficient random integration and targeted genome modification in maize and sorghum”. While the paper may not mention turfgrass in it, my interest was sparked because the authors were utilizing new methods to do plant transformations in monocots. They had great success with their methods and were able to achieve some impressive feats not normally seen in plants. When I got to the last figure in the paper, however, is when I got very excited. The figure, shown below, showcases proof that their new method works in a several monocot species and not just maize or sorghum. After seeing this, I believed that the method had the potential to work in turfgrasses, as they are a species quite closely related to some of the species shown like rice and switchgrass.
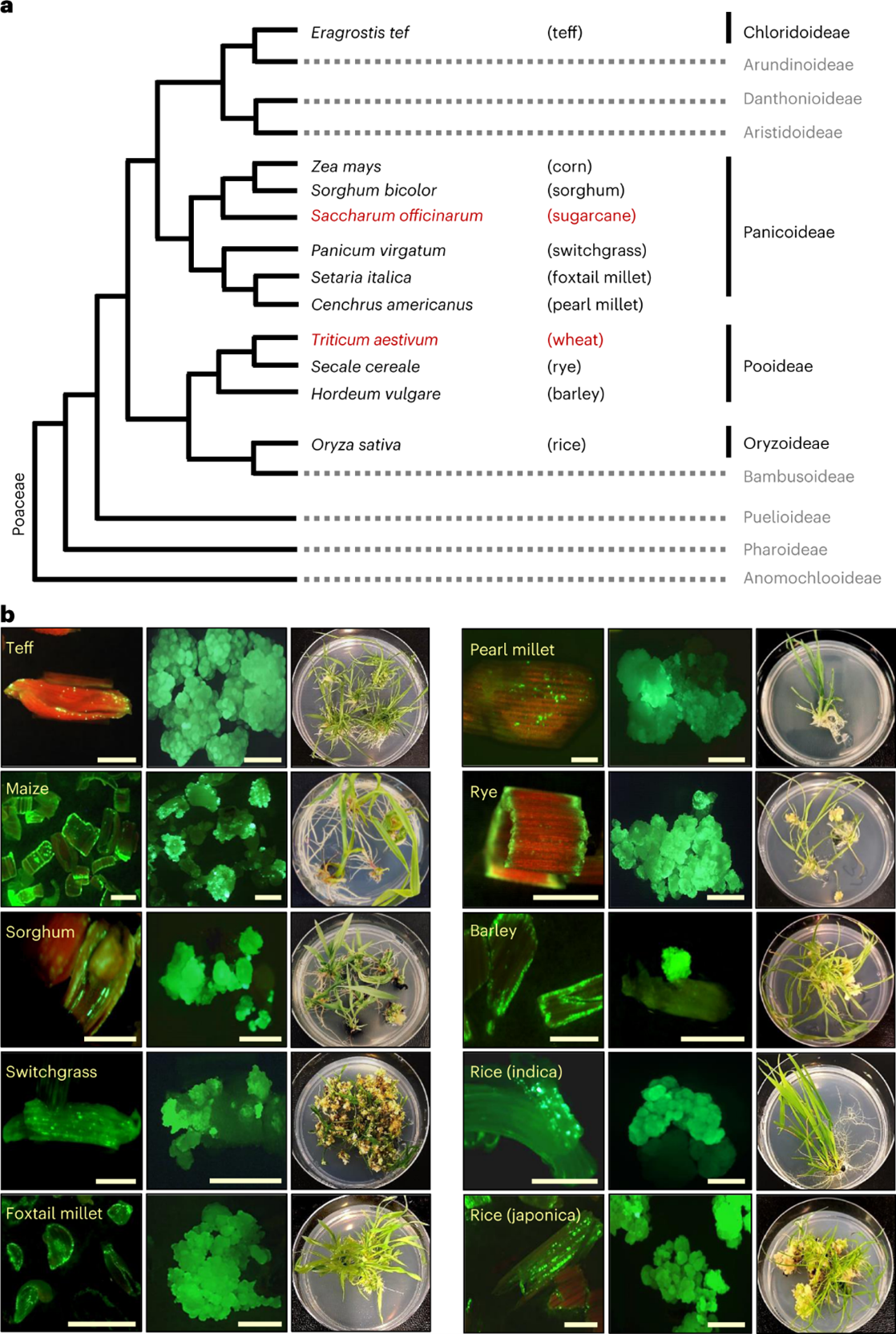
The new method revolves around the insertion of three genes. Wus, Bbm, and Cre. Wus and Bbm are both genes involved in embryogenesis, meaning that they are active and functioning in the areas of the plant that are actively growing, and play major roles in the formation of new plant tissue. In the past, they have been shown to do two things: boost regeneration rates in callus culture when transformed into the cells (Pan C. 2022); and induce formation of new plants by injecting Agrobacterium carrying the genes into a plant (Maher M. 2020). In addition, the authors also included a Cre gene being driven by a Heat Shock Protein (HSP) promoter. This gene is a recombinase, which allows for the excision of the Wus, Bbm, and Cre genes after they are no longer needed. Because the HSP promoter only gets activated under high temperatures, researchers can then control when the Wus, Bbm, and Cre genes are excised based on when they put the callus in high heat. This means that researchers can transform a plant with genes that help embryonic callus grow easily, and then when that process is complete, the temperature can be raised and the inserted callus forming genes, which are no longer necessary, will be removed and the cells can begin rapidly regenerating into a plant.
This is incredibly useful for a number of reasons:
- The process is not as reliant upon the plants own genotype to induce callus formation, since the genes to form this callus is being transformed in
- This process directly transforms tissue from seedlings, instead of having to go through a months long process of generating callus before it can be transformed.
- The Cre gene allows for the removal of unnecessary genes after they have completed their needed function.
- The overall approach results in far higher rates of transformation than other methods
With such promising results showcased in this paper, I wouldn’t be surprised at all to see parts of if not all of it become standard in plant genetic modification protocols. I will soon be testing the methods out in perennial ryegrass and potentially other species of turfgrass. Stay tuned to hear more in the future.
References
Maher, Michael F et al. “Plant gene editing through de novo induction of meristems.” Nature biotechnology vol. 38,1 (2020): 84-89. doi:10.1038/s41587-019-0337-2
Pan, C., Li, G., Malzahn, A.A. et al. Boosting plant genome editing with a versatile CRISPR-Combo system. Nat. Plants 8, 513–525 (2022). https://doi.org/10.1038
Wang, N., Ryan, L., Sardesai, N. et al. Leaf transformation for efficient random integration and targeted genome modification in maize and sorghum. Nat. Plants 9, 255–270 (2023). https://doi.org/10.1038/s41477-022-01338-0